Explain Equivalence Point and End Point
4 rows A point of equivalence in a titration refers to a point at which the added titrant is. Endpoint may or may not be the end of titration but equivalence is the complete end of titration after resulting in change of color of solution.
Difference Between Endpoint And Equivalence Point Difference Between
This is the main difference between equivalence point and endpoint.
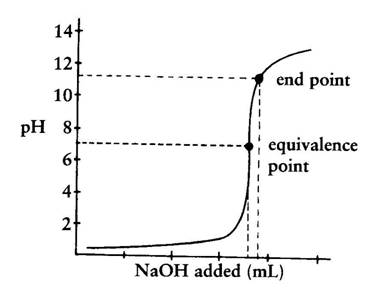
. If colour change of indicator is occurred at pH7 in strong acid - strong base titration. This is the equivalence point halfway up the steep curve. Titration end point.
Using the endpoint to calculate equivalence naturally introduces error. Explain how the pH at the equivalence point varies as the strength of the acid increases. Explain the difference between the equivalence point and the end point.
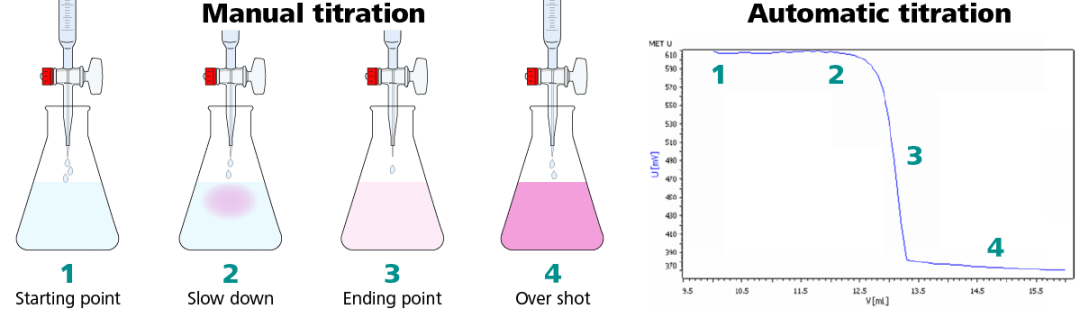
Methods of Determining the Equivalence Point. However very often we can easily spot a point very close to the equivalence point - and thats where the end point will be. Equivalence point in a titration is the point at which the added titrant is chemically equivalent completely to the analyte in the sample whereas the end point is the point where the indicator changes its colour.
Indicators are used to find pH value in equivalence point. Equivalence point is the point where equal number of moles of acid and the number of moles of base that have been mixed together are equal. So the difference between the equivalence point of dehydration and the endpoint the easiest way to understand it is that the equivalence point is just a theoretical point that you would like figure out on your paper in calculations theoretical and then the endpoint is something that you would actually observed in the lab.
1 Explain the difference between equivalence point and end point. The equivalence point is the precise limit where the chemical reaction stops in the titration combination. End point is the point at which the indicator being used in such a reaction changes colour.
Your answer should be based upon the proton transfer reaction that is important at the equivalence point. The term end point is where the indicator changes colour. At this point moles of NaOH added moles of HCl in the analyte.
Endpoint is the point in the titration where the indicator changes its color. The endpoint refers to the point at which an indicator changes color. That colour changeing point is called end point.
If the volume of the original vinegar sample was 500 ml calculate the mass of acetic acid in the vinegar. Remember that acid strength depends upon Ka not upon analytical concentration. More often than not the color change occurs after the equivalence point has already been reached.
The term equivalence point means that the solutions have been mixed in exactly the right proportions according to the equation. The stage occurs before the endpoint which signals the completion of the reaction. Provides that point where the unknown analyte has completely reacted with titrant and reactions ends.
For instance if you have 1 mole of acid and you add 05 mole of base exactly half of the acid will. The end point is where the titration ends in practice. Endpoint and stoichiometric point in common equivalence point are always different from each other.
In a typical titration a student needed 3648 mL of 01067 M NaOH to reach the phenolphthalein endpoint. This is the pH recorded at a time point just before complete neutralization takes place. At this point H O ions are completely neutralized by OH ions.
Colour of indicator is changed at one range of pH. The key difference between endpoint and stoichiometric point is that endpoint comes just after the stoichiometric point whereas stoichiometric point is the most accurate point at which the neutralization completes. In some cases there are multiple equivalence points which are multiples of the first equivalent point such as in the titration of a diprotic acid.
The term neutral point is best avoided. The closer the end point to the equivalence point the better but it is often not easy to find a good method of equivalence point detection. If we use the endpoint to determine equivalence it will induce an error.
Equivalence point is reached when Reactants react at Stoichiometric ratios and reach the Endpoint so that no more of the solution being titrated is foundEg. The endpoint is referred to as the point at which the used indicator changes its color. Equivalence point can be multiple in number during titration but the endpoint is the one point and does not occur frequently.
Equivalence point or stoichiometric point occurs during a chemical titration when the amount of titrant added is equivalent or equal to the amount of analyte present in the sample. These color changes appear after reaching the equivalence point. The equivalence point is not the same as the endpoint of a titration.
If you are titrating an acid against a base the half equivalence point will be the point at which half the acid has been neutralised by the base. The equivalence point is where the amount of moles of acid and base are equal resulting a solution of only salt and water. Equivalence point also called stoichiometric point in a nutshell is a point where the moles of the two solutions acid and base are equivalent or equal.
As you will see on the page about indicators that isnt necessarily exactly the same as the equivalence point. Strong base Strong Acid. The endpoint is the point where the color change occurs in the arrangement.
The equivalence point cannot be taken the same as the endpoint of a titration. Moreover the equivalence point always comes before the endpoint of the titration.
Difference Between Endpoint And Equivalence Point Difference Between
Endpoint Vs Equivalence Point What S The Main Difference Psiberg

Titration Curves Equivalence Point Article Khan Academy
Difference Between Equivalence Point And Endpoint Definition Properties Examples
Difference Between Equivalence Point And Endpoint Definition Properties Examples

Types Of Flasks And Their Uses In Chemistry Diagrams Examples Pedia
What Is The Difference Between A Half Equivalence Point And An Equivalence Point In Chemistry Quora
Difference Between Equivalence Point And Endpoint Definition Properties Examples

Equivalence Point Labster Theory
Endpoint Vs Equivalence Point What S The Main Difference Psiberg

Difference Between Endpoint And Stoichiometric Point Compare The Difference Between Similar Terms
Difference Between Endpoint And Equivalence Point Difference Between
Endpoint Vs Equivalence Point What S The Main Difference Psiberg
Endpoint Vs Equivalence Point What S The Main Difference Psiberg
Endpoint Vs Equivalence Point What S The Main Difference Psiberg
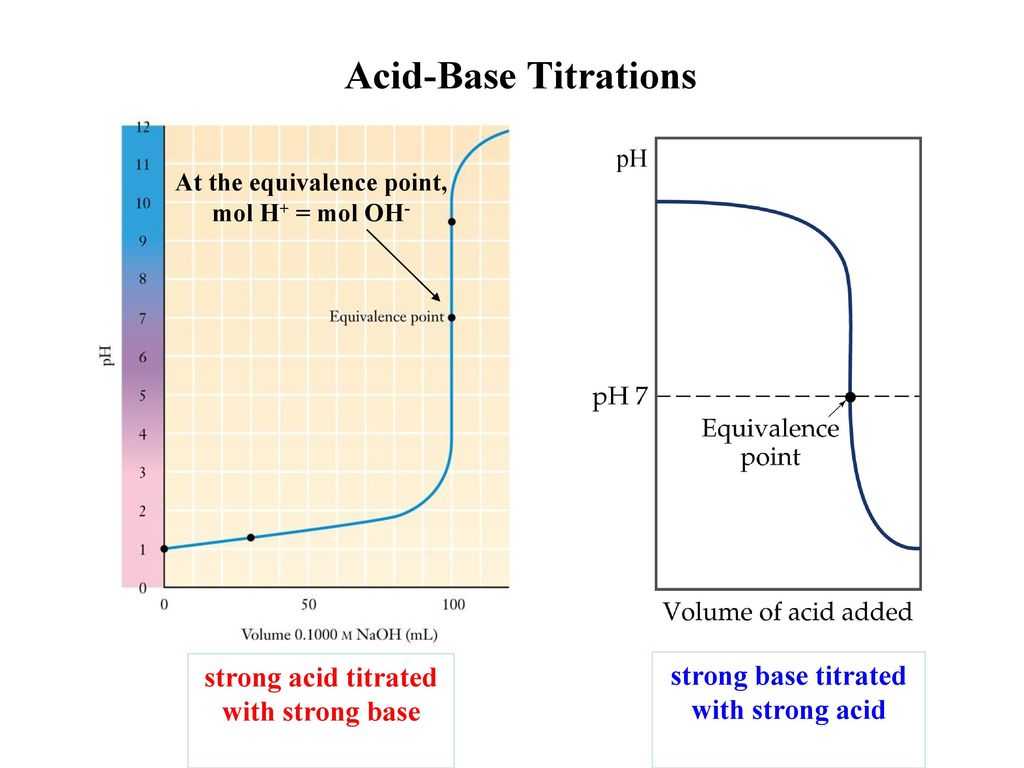
Acid Base Titrations End Point And Equivalence Point Ppt Download
Comments
Post a Comment